From the Desk of Ronald W. Davis, Ph.D.,
Chair of Open Medicine Foundation (OMF) Scientific Advisory Board
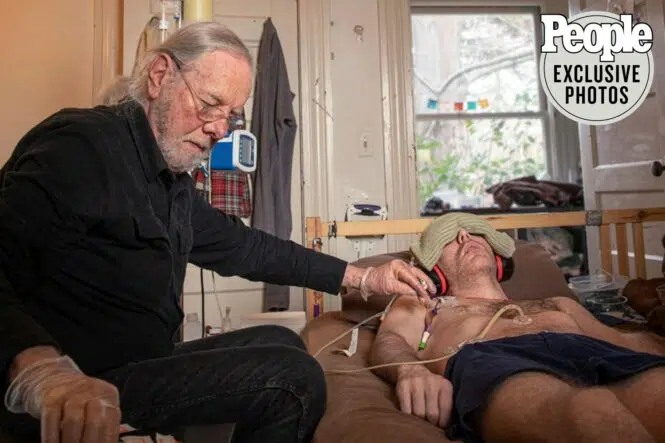 I am honored to share that my research into Myalgic Encephalomyelitis / Chronic Fatigue Syndrome (ME/CFS) has been featured by People Magazine! This feature details my family’s personal struggle to find treatments or a cure for my son Whitney, who has suffered from severe ME/CFS for over a decade.
As stated in Peoples’ teaser article, I don’t care who solves ME/CFS. I just know that it can be solved using good reasoning and good experiments. There’s something broken here, and I know we can fix it.
We hope this feature can increase large-scale awareness of the devastation ME/CFS causes and will attract new funding opportunities and researchers to the cause.
I am honored to share that my research into Myalgic Encephalomyelitis / Chronic Fatigue Syndrome (ME/CFS) has been featured by People Magazine! This feature details my family’s personal struggle to find treatments or a cure for my son Whitney, who has suffered from severe ME/CFS for over a decade.
As stated in Peoples’ teaser article, I don’t care who solves ME/CFS. I just know that it can be solved using good reasoning and good experiments. There’s something broken here, and I know we can fix it.
We hope this feature can increase large-scale awareness of the devastation ME/CFS causes and will attract new funding opportunities and researchers to the cause.
Read the teaser in People Magazine now.
(Be sure to look for the full print version, now available in stores, or in digital as soon as it is available!)
People magazine will also be airing a TV show featuring me and my family to accompany the teaser article on April 5, 2021 starting at 7pm ET / 4PM PT. You can stream the show on April 5 at 7pm ET here. You can also watch the show on People.com, PeopleTV app and People’s Facebook, Twitter and YouTube accounts. If you can’t tune in on April 5, the feature will be made available for later viewing here.I would also like to share with you a video update about exciting research progress happening at the ME/CFS Collaborative Research Center at Stanford!
This video is about our successful demonstration of the Metabolic Trap in yeast. We inserted the Human IDO1 gene into Baker’s Yeast. This gene leads to the production of a chemical compound called Nicotinamide Adenine Dinucleotide (NAD), essential for yeast to grow. We eliminated all other ways for yeast to produce NAD. Under normal levels of Tryptophan, the yeast grows normally.
When we increased the Tryptophan, the yeast stopped growing. When we reduced the Tryptophan, the yeast resumed growth. This is a demonstration of the Metabolic Trap in an organism! This is exciting because it provides us with an easy means to test if various drugs and/or supplements might get the yeast out of this Metabolic Trap, providing the possibility that this might work in humans.
We are proceeding to try this in human cells. I want to give special acknowledgment and gratitude to Open Medicine Foundation because the early work analyzing the data from the Severely Ill Patient Study led to the Metabolic Trap hypothesis. This work was largely funded by Open Medicine Foundation and we couldn’t have done it without them.
Lastly, I am excited to share this paper written by Laurel Crosby Ph.D., Dr. Hector Bonilla, and colleagues at Stanford University. I believe this publication details a potentially very important development in the treatment of ME/CFS.
Dr. Hector Bonilla started prescribing low dose Abilify to some of his patients based on a report from a patient that it had helped her.
He began to find that it significantly improved the symptoms of many patients, with few side effects, so he and Laurel Crosby, Ph.D., and colleagues, decided to do a retrospective examination of his patient records. We were all impressed with the findings and thought it important to publish this very preliminary report. We are pleased to have now established a good working relationship with the Stanford ME/CFS Clinic, led by Dr. Bonilla, and we are now pursuing Abilify for a placebo-controlled, double-blind study to more formally determine if Abilify should become an FDA approved treatment for ME/CFS.
Having witnessed the significant improvements that my son, Whitney Dafoe, has experienced with Abilify, I’m hopeful that other patients will be able to benefit as well.
Read the full paper on Abilify
*Please note – OMF funds research projects at our five established Collaborative Research Centers (CRC). We are not typically involved in the research process. Each CRC operates independently and recruits research participants from clinicians that they collaborate with locally. CRCs are not able to respond to inquiries from the general public. Additionally, OMF is not authorized to give medical advice or comment on personal medical concerns.*
Help us keep the research going.
Together we can improve the quality of life for all sufferers of ME/CFS and other chronic complex diseases, such as Post Treatment Lyme Disease Syndrome and Fibomyalgia.
Donate to Open Medicine Foundation today!